THIS IS HOW RING OF 18 CARBONS IS MADE : A RESEARCH DONE BY THE UNIVERSITY OF OXFORD
Why a stable ring of cyclocarbon is a big deal
Carbon can be arranged in a number of configurations and one such form is cyclo[n]carbon, which is a ring of carbon atoms bonded to each consisting only of carbon atoms. Carbon through allotropy, the property of elements to exist in two or more forms in the same physical state, can exist as diamond, graphite, fullerene and other forms with different physical and chemical properties.
When each of carbon atoms in the ring is bonded to three other carbon atoms, it’s relatively soft graphite, whereas, with the addition of just one more bond, it becomes one of the hardest minerals known– diamond. When 60 carbon atoms are bonded together in a soccerball shape, you get buckyballs.
However, a ring of carbon atoms where each atom is bonded to just two other carbon atoms was not achieved until now as they could not be isolated or structurally characterized, due to their high reactivity. The past attempts had resulted in gaseous carbon ring that dissipates as soon as they were formed.
How they managed to pull this off
Researchers were able to produce the C18 compound by eliminating carbon monoxide from a cyclocarbon oxide molecule C24O6– the triangular cyclocarbon oxide compound where 18 carbon atoms are bonded to six carbon monoxide. They used atom manipulation as they transferred this concoction to a layer of sodium chloride (NaCl) on a copper plate (Cu), chilled in a vacuum chamber at 5 Kelvin.
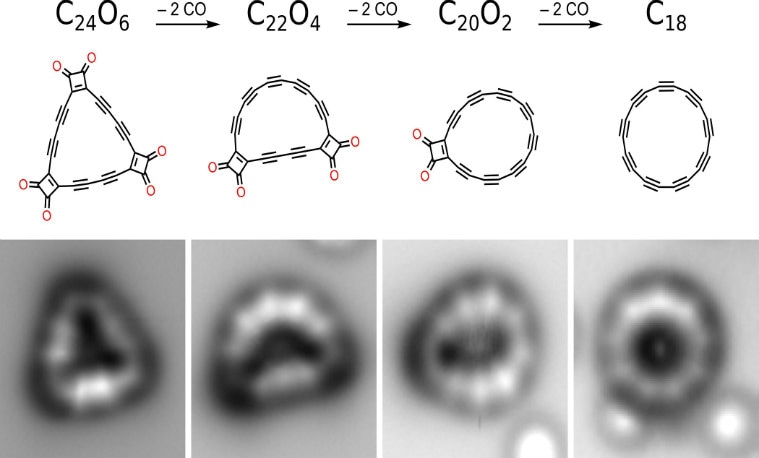
This provided an inert surface that kept the structure stable where the compound was formed by eliminating carbon monoxide (CO) molecules off the structure, leaving just the ring of carbon atoms behind with a polyynic structure of carbon atoms with an alternating triple and single bonds.
Removing the scaffolding to make the ring is not so simple as it sounds. “We removed all six CO moieties from C24O6, with 13 per cent yield, typically resulting in circular molecules,” the researchers wrote in their paper.

Comments
Post a Comment